Statins: Saviours of Mankind or Expensive Scam?
I cannot claim credit for this article. It is an amalgam of two others by Dr Uffe Ravnskov, for the most part, and a statistician, Al Lohse. My only contribution is putting the two together in a way, I hope, that makes the whole more meaningful to readers wanting to know the facts about statins.
Introduction
Although there is not, and never has been, any convincing evidence that levels of serum cholesterol have any causal relationship with coronary heart disease, that hasn't stopped the cholesterol hypothesis being used as a basis for the sale of drugs to lower cholesterol.
Over the last half of the twentieth century a whole range of drugs were tried. All, without exception, were less than successful. And no evidence was produced that cholesterol-lowering, whether by diet or various older drugs such as clofibrate, gemfibrozil, cholestyramine, colestipol, or nicotinic acid, extends life or reduces overall mortality.
But the new type of cholesterol-lowering drugs called statins do appear to be successful. There is no doubt that in trials there has been a reduction in the numbers of deaths among those taking statins compared to control groups.For the first time cholesterol-lowering has shown significant improvement in mortality rates, from coronary mortality, stroke mortality and total mortality.
Statins are now the drugs of choice and aggressively marketed. As these cost around £400 per person per year, and they have to be taken for life, the drug companies can look forward to several years of very healthy profits until the patents run out.
So statins increase the health of drug companies' bank balances, but do they really increase the health of those who take them? And do they represent good value for money as far as a cash-strapped National Health Service is concerned?
Basis for the guidelines
All of the current guidelines are based on a series of large, clinical trials testing lipid control agents — for the most part statins — against the absence of trial drug. Two early lipid-lowering trials were the Scandinavian Simvastatin Survival Study (4S), with simvastatin, and the Cholesterol and Recurrent Events (CARE) trial, with pravastatin. The primary finding of both these trials was that lowering LDL cholesterol in patients at risk for CVD to within the range advocated by subsequent guidelines would significantly decrease the number of deaths from coronary artery disease. Subsequently, both the West of Scotland Coronary Prevention Study (WOSCOPS) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) found that using a statin to lower plasma cholesterol levels produced a significant reduction in coronary events, even in subjects without clinically evident CVD.
The effect of the statins is not due to cholesterol-lowering
But there are some questions about the results of trials into statins and what exactly their mode of action is because, although trial directors concluded in their reports that reducing total and LDL cholesterol was statins' mode of action, some of the results were not consistent with what we know about cholesterol.
First, the statins were effective for women. This is a surprising finding because most studies have shown that high serum cholesterol is not a risk factor for women.
Second, older individuals were protected just as much as young ones, although most studies have shown that high serum cholesterol is a weak risk factor, or no risk factor at all, for men above fifty, and actually increases longevity in those over eighty.
Third, the number of strokes was reduced after statin treatment, although no studies have shown that high serum cholesterol is a risk factor for stroke.
Fourth, patients who have had a myocardial infarct were protected although most studies have shown that high serum cholesterol is a weak risk factor, if any at all, for those who already have had a myocardial infarct.
And finally, the statins protected against coronary heart disease whether the cholesterol was high or low although most studies have shown that a normal or low cholesterol level is no risk factor for coronary disease.
So, if cholesterol level for these people is not a risk factor for coronary disease, how could lowering that cholesterol improve their chances to avoid a coronary? The only reasonable explanation is that the statins do more than just lower cholesterol.
There is much evidence for this.
Other effects of statins
The statins inhibit the body's production of mevalonate, which is a precursor of cholesterol. When the production of mevalonate goes down, less cholesterol is produced by the cells and thus blood cholesterol goes down as well.
But mevalonate is a precursor of other substances also, substances with important biologic functions. The metabolic pathways are not known in all details, but less mevalonate may explain why simvastatin makes smooth muscle cells less active and platelets less inclined to produce thromboxane A 2 . One of the first steps in arteriosclerosis is the growth and migration of smooth muscle cells inside the artery walls — and thromboxane A 2 is a substance which promotes the clotting of blood. Thus, by blocking the function of smooth muscle cells and platelets, simvastatin may benefit cardiovascular disease by at least two mechanisms both of which have nothing to do with serum cholesterol levels.[1]
In one of the experiments, performed by Dr. Yusuke Hidaka and his team, the inhibitory effect on the muscle cells could not be abolished by adding LDL-cholesterol to the test tubes[2] and in experiments with various cholesterol-lowering agents, thromboxane A 2 production was inhibited by statins only, indicating that the effect was not due to cholesterol lowering but to something else. 1
The protective effects of simvastatin were also demonstrated in animal experiments. In one of them, performed by Dr. B.M. Meiser and colleagues from Munich, Germany, hearts were transplanted into rats. Normally, the function of such grafts gradually deteriorates because the coronary vessels are narrowed by an increased growth of smooth muscle cells in the vascular walls, a condition called 'graft vessel disease'. In Meiser's experiment, however, rats that were given simvastatin had considerably less graft vessel disease than control rats not given simvastatin. This was not due to cholesterol lowering because simvastatin does not lower cholesterol in rats. In fact, LDL cholesterol was highest in the rats treated with simvastatin.[3]
In another experiment, Dr. Maurizio Soma and his colleagues from Milan, Italy, placed a flexible collar around one of the carotid arteries in rabbits. After two weeks arteries with collars became narrow but less so if the rabbit had been given simvastatin. Again, the effect was unrelated to the rabbits' cholesterol level.[4]
Thus, the statins in some way protect against cardiovascular disease, but their effect is not due to cholesterol-lowering .
But why bother about pharmacological mechanisms? Isn't it wonderful that the statins work? Shouldn't we all take statins?
But just how effective are statins — really?
In trials of statins the results are stated in terms of 'relative reduction' in events. This can be highly misleading. It is much more honest to provide the figures that accurately represent the chances of a patient surviving with and without treatment.
When releasing its report on the Heart Protection Study to the press, the Medical Research Council announced it as a 'Life-saver' with headlines saying 'World's largest cholesterol-lowering trial reveals massive benefits for high-risk patients.'[5] In fact the absolute reduction in risk of dying from any cardiovascular diseases in this trial was a mere 3.1% (Table 1). It was also substantially less than in the previous Scandinavian simvastatin survival study (4S)[6]
|
|
HPS |
4S |
|
CHD mortality |
1.2 |
3.5 |
|
Total mortality |
1.7 |
3.3 |
|
All stroke |
1.5 |
3.5 |
|
Any major CHD |
3.1 |
6.7 |
Table 1: Absolute risk reduction (%) in two trials of simvastatin
To report relative values as opposed to absolute figures gives the public a totally false impression of the real benefits of such treatment. What looks like a massive and highly significant probability for a long life, may not be.
The benefits of statins are less than impressive
There have been six large trials into statins. Total cholesterol was lowered by impressive amounts (in relative terms) but the reduction in numbers of total deaths was much less impressive ranging from a reduction of 3.3% in a trial of simvastatin[7] to an increased death rate of 0.3% in the CARE lovastatin trial.[8]
Statins and cancer in trials
In 1996 Drs Newman and Hulley of the University of California, San Francisco, published the results of a meticulous review of cholesterol-lowering drugs with reference to cancers. They found that 'all members of the two most popular classes of lipid-lowering drugs (the fibrates and the statins) cause cancer in rodents, in some cases at levels of animal exposure close to those prescribed for humans.'[9] Indeed, human trials have also demonstrated increases in cancers. Looking at the CARE trial, for example, we see a benefit in terms reductions in fatal heart disease deaths of 1.1% — but an increase in breast cancer deaths of 4.2%. These are absolute risk figures. The media and trial doctors like to report their findings in relative terms — these figures are more impressive in the media. If we look at the figures in this way, it translates into a modest 12% fewer fatal heart deaths, but a huge 1,400% increase in numbers of breast cancers.[10] The figures are shown in Table 2.
|
|
Number of patients in the pravastatin group |
Number of patients in the control group |
Relative risk with statin
|
Absolute risk with statin |
|
Death from a heart attack |
96 of 2081
|
119 of 2078
|
-12% |
-1.1% |
|
Cases of breast cancer |
13 of 290
|
1 of 286
|
+1,400% |
+4.2% |
Table 2: Fatal heart attacks and breast cancer rates. Data from the CARE trial.
And are the statins really safe in practice?
Although statins appeared to be completely safe in clinical trials, this has not been the finding in practice. Baycol, the statin made by the German company, Bayer, was taken off the market following a number of deaths due to a muscle wasting condition called rhabdomyolysis . But all statins list this as an adverse effect in their paperwork. Another notable side effect of statin therapy is myopathy. A small fraction of patients who are treated with statins will develop severe myopathy.[11] In the worst cases, severe myoglobinuria, acute renal failure, and even death can occur. Predisposing factors for severe myopathy appear to include advanced age, relatively low body weight, female sex, certain medications, use of multiple medications, multisystem disease, and acute illnesses or major surgery.
How accurate are the figures?
And just how accurate are the figures? The Scandinavian Simvastatin Survival Study represents the best results one might expect to get from a course of statin treatment. The differences were feeble anyway, but might they have been tampered with?
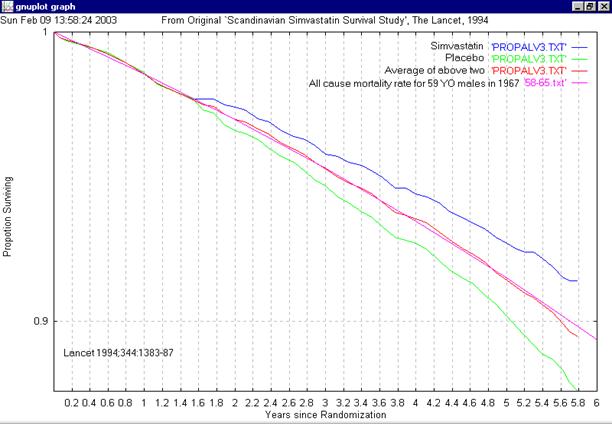
Let me point out the finer details of the graph:[12]
The treatment and placebo groups' mortality lines should be independent: a trend in one should have no consequential influence on the other. However:
- All 4 lines are essentially identical for 1.6 years.
- Then there is a departure — by both lines at the same time.
The fact that both lines — treatment and placebo — depart at the same time is important. Why should the treatment suddenly become beneficial at exactly the same time as non-treatment becomes detrimental?
The average line of both treatment and non-treatment groups follows a 'natural' mortality curve; any natural survival curve would have its slope increasing downward. (i.e. becoming more negative.)
Both treatment and placebo lines follow this natural curve for 1.6 years. Then both diverge. The placebo group shows this slope change increasing (negative) at a faster rate than all other lines. But, surely, it should follow the natural mortality curve. Why doesn't it?
The slope of the treatment group is nearly constant from 1.8 years onward. It's not a curve at all, but an almost straight line — and it shouldn't be. What it says is that old people die at the same rate as younger ones. And life isn't like that.
Is this evidence that the data of the 4S trial were not handled in an honest manner? Were deaths occurring in the treatment group assigned to the placebo group? Is this why the two curves, which should be independent, are apparently related? Or is there a mistake somewhere? Is there an error in logic?
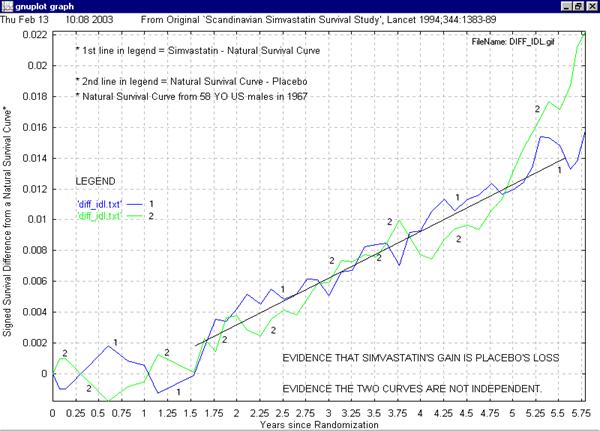
This second graph demonstrates that a transfer of the order of 7 deaths per year from the treatment group to the placebo group may have occurred. There is no certainty in statistics, but it certainly appears that the differences between the given curves (In Lancet 1994; 344: 1383-89) and a natural, all cause, survival curve, could easily be accounted for by the transfer of mortality data from one group to the other.
Now, I must admit, the Natural Survival Curve, appears to have been stumbled on serendipitously. However, when it essentially matches the mean (average) curve it means that the mean curve is 'well behaved' compared to the other two.
The other two diverge from one another and the mean and the natural curves at about 1.6 years. The mean curve remained 'well behaved'. Therefore, it is most likely the other two are not 'well behaved'. It would have been more likely that the treatment and placebo curves diverged from the natural curve at different times. To diverge at the same time is suspicious.
The other two curves are not independent of one another. Roughly speaking, this graph demonstrates that simvastatin and placebo curve differences may be entirely accounted for by the transfer of mortality data from one group to the other.
It does not prove that conclusively. The proof would lie in whether or not Simvastatin can be found in the bodies of individuals who were buried as 'placebos' as much as fifteen years ago.
The costs
But, if statins did work as well as they reportedly do, would they be worth paying for?
To answer that question it is necessary to look at the figures from the trials. To be short I have chosen the figures for coronary death. According to the results from the 4S trial there was a 41% reduction in the risk of coronary death.[13] According to the results from the CARE trial the reduction was 24%, 8 and according to the WOSCOPS trial the reduction was 28%.[14] These figures seem impressive, but let us look at the absolute figures also.
In the treatment group of the 4S trial five percent, or 111 individuals, died from a heart attack; in the control group 8.5 percent, or 189 individuals, died. This is a difference, or a risk reduction of 3.5%. To prevent these 3.5% of the patients (8.5% - 5%) or 78 individuals, from dying it was necessary to treat 2221 individuals for five years. You could also say that to prevent one death it was necessary to treat 25 individuals for five years. Or to put it another way, if you have had a heart attack the chance to avoid death from a new one during five years is 91.5%. If you take simvastatin this chance increases to 95%.
In the CARE trial 5.7%, or 119 individuals died from a heart attack in the control group and 4.6%, or 96 individuals in the treatment group. Thus, to prevent 23 coronary deaths (1.1%) it had been necessary to treat 2,081 individuals for five years, which means that 90 patients were treated for each life saved.
In the WOSCOPS trial, which concerned healthy individuals with a high cholesterol, the result was even less impressive. Here, 61 died in the placebo group, 41 in the treatment group, a risk reduction of 0.6%. To save these 20 lives it had been necessary to treat 3,302 healthy individuals for five years, or 165 individuals for each life.
In other words, if you are about 55 years old and your cholesterol is around 7.0 mmol/l, the risk of dying from a heart attack during five years is 1.8%. With pravastatin treatment the risk is reduced to 1.2%. You could also say that the chance to avoid death from a heart attack for five years is 98.2%; with pravastatin the chance is 98.8%.
The reason why trial results should be given in absolute figures and not in relative is because the side effects are given in absolute figures. Let us assume that a mortal side effect occurs in 0.5 percent of the patients. You may belittle that if you compare this figure for instance with a relative risk reduction of 28%. But as the absolute risk reduction was 0.6% the effect of treatment has almost disappeared.
To be fair I should mention that the number of non-fatal heart attacks was reduced also. In the WOSCOPS trial for instance, 248 individuals in the control group had a fatal or non-fatal coronary, in the pravastatin group the number was 174. This means that to prevent a heart attack in a healthy 55-year-old man with a high cholesterol level it is necessary to treat about 45 men for five years. To prevent a new heart attack it is necessary to treat 34 patients for five years according to the CARE trial and 28 patients according to the 4S trial.
So to look at the costs is not an easy task. For the drugs only the price for one extra year for one person was about £25,600 in the 4S trial, about £92,500 in the CARE trial and about £128,000 in the WOSCOPS trial. To that should be added the costs for laboratory tests and doctors' fees.
There are also economical gains, of course. The directors of the most successful trial, 4S, claim that the reduced costs due to the lower number of non-fatal heart attacks outweigh the expenses. But that trial concerned patients at a very high risk of cardiovascular disease. To treat healthy individuals with high serum cholesterol will be very expensive because the gain is very small.
Unwarranted assumptions?
The 4S directors' optimistic views presuppose that the effect is just as positive after ten or twenty years of treatment as it was after five. But there is no guarantee of that. Don't forget Newman and Hulley's published results from a meticulous review of what we know about cancer and lipid-lowering drugs mentioned above. They found that clofibrate, gemfibrozil and all the statins stimulate cancer growth in rodents. 9
Newman and Hulley asked themselves why these drugs had been approved by the Food and Drug Administration at all. The answer was that the doses used in the animal experiments were much higher than those recommended for clinical use. But as Drs. Newman and Hulley commented, it is more relevant to compare blood levels, and the levels achieved in rodents were very close to those seen in patients.
Because the latent period between exposure to a carcinogen and the incidence of clinical cancer in humans may be 20 years or more, the absence of any controlled trials of this duration means that we do not know whether statin treatment will lead to an increased rate of cancer in coming decades.
Thus, millions of asymptomatic people are being treated with medications, the ultimate effects of which are not yet known. Drs. Newman and Hulley, therefore, recommended that the new statins should be used only for patients at very high risk for coronary disease and that this treatment should be avoided for individuals with life expectancies of more than 10 to 20 years. Healthy people with a high cholesterol as the only risk marker belong to that category.
Other adverse effects?
Mevalonate, inhibited by statins, is the precursor for the biosynthesis of Coenzyme Q10. This has ramifications for other serious conditions.
CoQ10 is a deceptively simple molecule which lies at the centre of mitochondrial ATP production and appears to have clinically relevant antioxidant properties manifested by tissue protection in settings of ischaemia and reperfusion. Congestive heart failure has served as a model for measurable deficiency of CoQ10 in blood and tissue, which when corrected, results in improved myocardial function. Ischaemic heart disease, anginal syndromes, and most recently the ischaemia reperfusion injury of coronary revascularisation, have provided clear evidence of clinically relevant antioxidant cell protective effects of CoQ10. Newer P31 NMR spectroscopy studies such as those conducted in Philadelphia have documented enhanced cellular high energy phosphate concentrations with CoQ10 supplementation in models of ischaemia and reperfusion.[15] Sophisticated biochemical markers of oxidative injury are now demonstrating in-vivo the antioxidant cell protective effects of CoQ10.
The heart muscle requires more ATP than any other tissue and accordingly has a huge concentration of mitochondria — approximately one third of the volume of heart muscle is mitochondria, far more than any other cell type and with the high proportion of mitochondria, the heart muscle also has the highest level CoQ10. Heart muscle is thereby uniquely vulnerable to CoQ10 depletion or deficiency. Beginning at about the age of 25-30, biosynthesis of CoQ10 begins to decline and blood and tissue levels have been documented to steadily fall after this age.
Peter H. Langsjoen, M.D., F.A.C.C. and Alena M. Langsjoen, M.S reviewed 30 years of clinical publications on CoQ10 and combined it with their own clinical experience.[16] It was clear that there were several consistent and unique characteristics of the clinical effects of CoQ10 supplementation which are worthy of discussion which, for simplicity, they termed the 'Q effect'. The benefits of CoQ10 supplementation were likely not due solely to a correction of deficiency in so far as clinical improvements are frequently seen in patients with 'normal' pre-treatment CoQ10 blood levels and optimum clinical benefit requires above normal CoQ10 blood levels (2 to 4 times higher). They found that high blood levels might be required to attain an elevation of tissue CoQ10 levels or to rescue defective mitochondrial function perhaps by driving cytosolic glycolysis or the plasma membrane oxidoreductase or by directly enhancing the function of defective mitochondria.
But supplements of CoQ10 appeared to affect much more than just cardiac myocytes. Many aspects of their patients' health tended to improve in a way that could not be explained by the observed improvement in heart function.
CoQ10 does not lend itself to traditional organ-specific or disease-specific strategy and requires a reassessment and a rethinking of medical theory and practice. However, the fact that statins inhibit the natural biosynthesis of CoQ10 may prove damaging.
Some of the other known endproducts of the mevalonate pathway are prenyl proteins.
Another note of caution
To test a drug on many thousands of patients is extremely costly and laborious. The only groups willing to spend several hundred million dollars for such a trial are, of course, the drug companies because the potential for profits is enormous. Naturally, all of the statin trials were sponsored by the company whose drug was tested in the trial. Not only did the companies pay for the necessary meetings, workshops, conferences, speakers fees and travelling expenses for the many hundreds of participating doctors and researchers in each trial, they also offered assistance in the preparation of the trial, the selection of patients and control individuals, the construction and production of the protocols, the monitoring of the results, the cholesterol analyses and the complicated statistical calculations. Can we be totally confident that their vested interests had no influence at all on the outcome of these trials?
And were the results really blinded as we have been told? In most of the trials the lipid analyses were performed at the drug company laboratories, and these results were not released to the doctors and patients throughout the whole trial. But what about the lipid analyses that may have been performed at individual clinics — were they blinded also? When the first favourable results from the trial were announced in the press, for example, how would participants in the other trials react? Wouldn't they have wanted to know whether they were taking the new wonder drug or an ineffective placebo? One easy way to find out would be to take a cholesterol test. Almost certainly, all of them knew their cholesterol level at the beginning of the trial.
It is not unreasonable to assume that a substantial proportion of the patients and their doctors knew to which group the participant belonged and such information might have unintentionally influenced the results.
But let us assume that the doctors and the patients were not influenced at all. What about the trial directors? Was there pressure to exaggerate the trivial effects of the treatment and minimize side effects. Many of these reports do not appear to have been written by scientists in search of the truth and nothing but the truth.
Consider also that positive results are much more rewarding for researchers than negative ones. Researchers who come up with positive results, in particular positive results from drug trials, are more often invited as speakers to meetings and congresses and more often chosen for further lucrative research projects.
Can we, therefore, be confident that statin research results have been presented in a nonpartisan manner? Why, for instance, haven't we heard about the outcome of the first statin trial, the EXCEL study?
EXCEL, the Expanded Clinical Evaluation of Lovastatin
This trial was performed in a large number of American clinics and research institutions, including the Merck Sharpe & Dohme Research Laboratories at West Point, NY, where the drug was produced. More than 8,000 'patients' with cholesterol levels between 6.0 mmol/l and 7.8 mmol/l received one of four different doses of lovastatin (Mevacor) or a placebo.
With a view to reporting on possible adverse effects of the treatment, preliminary study results were published after only one year of the trial.[17] No significant side effects were reported, but in the fine print the authors were obliged to mention that death due to all causes was 0.5 percent in the four lovastatin groups combined (32 or 33 individuals out of a group of about 6,600 — no exact figures were given in the report) compared to 0.2 percent in the placebo group (three or four individuals out of a group of 1,650.) By taking all the lovastatin groups together, the difference would have been statistically significant if the number of deaths in the treatment groups were 33, but not if it were 32. But even if the difference were not statistically significant after one year, it would certainly have become significant if the tendency to a higher mortality in the treatment groups had continued throughout the trial. In any case, the aim of the treatment was to lower mortality and most certainly no lowering was achieved.
Today at least twenty reports from the EXCEL trial have been published in various medical journals. These reports tell us how well lovastatin is tolerated and how effective it is in lowering blood cholesterol levels in various populations. But not one of them has reported the final outcome of the trial, although more than ten years have passed since it began. Therefore, we do not know whether the increased mortality, seen after just one year of treatment, has continued throughout the trial.
Why, then, have we not heard about the outcome of this trial, the largest of them all? And are there more trials we haven't heard about? Or are there any unfavourable effects in the published trials that we haven't heard about either?[18]
It is safe to assume that lowering cholesterol with drugs is not a harmless enterprise.
Conclusion
Statins increase life expectancy in short-term trials, but not by very much. There are questions about the accuracy of trial results and the safety of statins in the long-term is not established. The cost-effectiveness of statins is also poor and the cost to the NHS for all those currently being suggested as needing them would be crippling.
Until a considerable amount of better evidence is available that demonstrate statins do have real, long-term benefits without serious adverse side effects, it might be wise to restrict their use to patients at very high risk.
References
[1]. Ravnskov U. Implications
of 4S evidence on baseline lipid levels.
Lancet
1995; 346: 181.
- Massy ZA, Keane WF, Kasiske BL. Inhibition of the mevalonate pathway:
benefits beyond cholesterol reduction?
Lancet
1996; 347: 102-103.
- Vaughan CJ, Murphy MB, Buckley BM. Statins do
more than just lower cholesterol.
Lancet
1996; 348: 1079-1082.
[2]. Hidaka Y, Eda T, Yonemoto
M, Kamei T. Inhibition of cultured vascular smooth muscle cell migration by
simvastatin (MK 733).
Atherosclerosis
1992; 95: 87-94.
[3]. Meiser BM, and others.
Simvastatin decreases accelerated graft vessel disease after heart
transplantation in an animal model.
Transplantation
Proceedings
1993; 25: 2077-9.
[4]. Soma MR, and others. HMG
CoA reductase inhibitors. In vivo effects on carotid intimal thickening in
normocholesterolemic rabbits.
Arteriosclerosis
1993; 13: 571-8,
[5]. Medical Research
Council/British Heart Foundation Heart Protection Study. Press release.
Life-saver: World's largest cholesterol-lowering trial reveals massive benefits
for high-risk patients. Available at www.ctsu.ox.ac.uk/~hps/pr.shtml
[6]. Scandinavian Simvastatin
Survival Study Group. Randomised trial of cholesterol lowering in
4444 patients with coronary heart disease: the Scandinavian simvastatin
survival study (4S).
Lancet
1994; 344: 1383-1389
[7]. Downs JR, et al. Primary
prevention of acute coronary events with lovastatin in men and women with
average cholesterol levels: Results off AFCAPS/TexCAPs.
JAMA
1998; 279:
1615-22.
[8]. Sacks FM and others. The effect of pravastatin on coronary events after
myocardial infarction in patients with average cholesterol levels.
New
England Journal of Medicine
1996; 335: 1001-1009.
[9].
Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs.
JAMA
1996; 275: 55-60
[10]. Sacks FM and others. The
effect of pravastatin on coronary events after myocardial infarction in
patients with average cholesterol levels.
New Engl J Med
1996;335:1001-1009
[11]. Staffa JA, Chang J, Green
L. Cerivastatin and reports of fatal rhabdomyolysis.
N Engl J Med.
2002;346:539-40.
[12]. Al Lohse, MEng.
(Statistician) Personal communication.
[13]. The Scandinavian
Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in
4444 patients with coronary heart disease: the Scandinavian Simvastatin
Survival Study (4S).
Lancet
1994; 344: 1383-1389
[14]. Shepherd J and others.
Prevention of coronary heart disease with pravastatin in men with
hypercholesterolemia.
New Engl J Med
1995; 333:1301-1307.
[15]. J.A.Crestanello,
J.Kamelgard, D.M.Lingle, S.A.Mortensen, M.Rhode, G.J.Whitman. Elucidation of a
tripartite mechanism underlying the improvement in cardiac tolerance to
ischemia by coenzyme Q10 pretreatment.
J Thorac Cardiovasc Surg
,
1996;111: 443-50.
[16]. Langsjoen PH, Langsjoen
AM. Overview of the Use of CoQ10 in Cardiovascular Disease.
BioFactors
1999; 9: 273-284. http://wwwcsi.unian.it/coenzymeQ/overview.htm
[17]. Bradford RH and others. Expanded clinical evaluation of lovastatin
(EXCEL) study results.
Arch Int Med
1991;151: 43-49.
[18]. Ravnskov asked that question of Merck, Sharp & Dohme. They answered
that the trial was not designed to measure the clinical outcome, only to test
whether the drug was tolerable and did not produce any serious side effects.
Related Articles

 HOME
HOME


